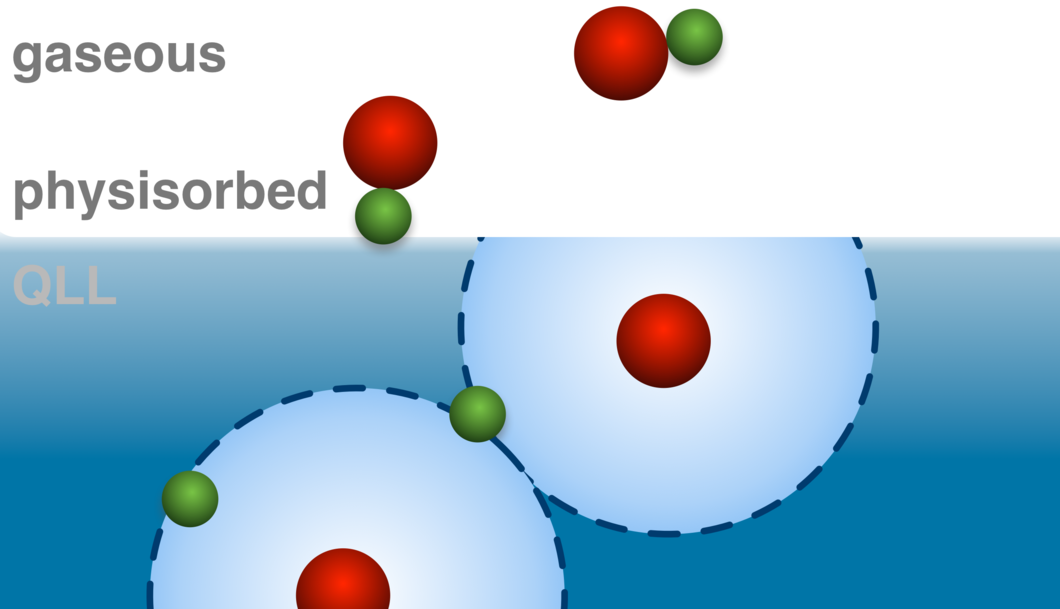
Coexistence of Physisorbed and Solvated HCl at Warm Ice Surfaces
The interfacial ionization of strong acids is an essential factor of multiphase and heterogeneous chemistry in environmental science, cryospheric science, catalysis research and material science. Using near ambient pressure core level X-ray photoelectron spectroscopy, we directly detected a low surface coverage of adsorbed HCl at 253 K in both molecular and dissociated states. Depth profiles derived from XPS data indicate the results as physisorbed molecular HCl at the outermost ice surface and dissociation occurring upon solvation deeper in the interfacial region. Complementary X-ray absorption measurements confirm that the presence of Cl− ions induces significant changes to the hydrogen bonding network in the interfacial region. This study gives clear evidence for nonuniformity across the air−ice interface and questions the use of acid−base concepts in interfacial processes.