Understanding of the Oxygen Evolution Reaction Kinetics in Acidic Environment
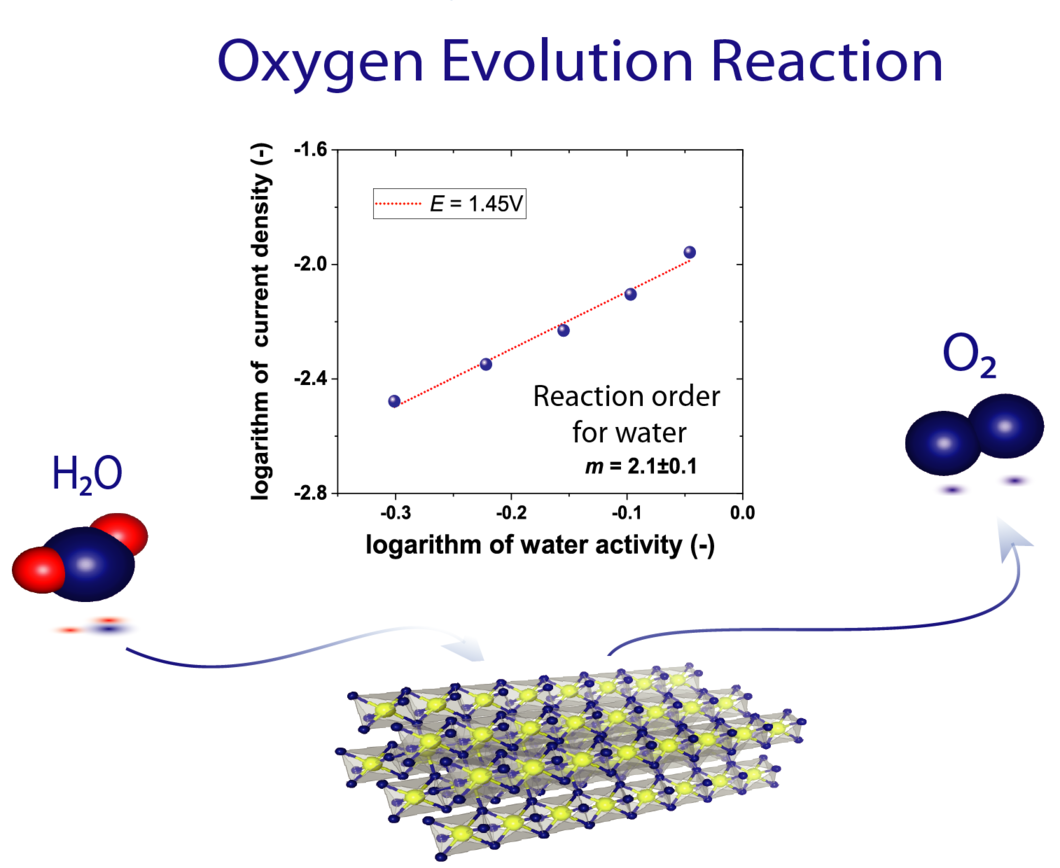
The high operational expenditure of polymer electrolyte water electrolysis (PEWE) technology, dominated by kinetic losses from the sluggish oxygen evolution reaction (OER), inhibits large-scale market penetration. PSI researchers have developed a novel methodology to access underlying reaction mechanism of the OER. For the first time the reaction order for water has been determined. Advanced benchmarking of catalysts in technical environment also supports the development of novel, highly efficient catalyst materials.
For rational design of highly active OER catalyst materials, the reaction mechanism (RM) and related reaction intermediates are of main interest. Over the last decades, several RM have been proposed but experimental evaluation has been still missing. In particular, the reaction order with respect to water, is essential for the identification of valid RM. Furthermore, benchmarking of OER catalyst with conventionally used characterization techniques based on liquid electrolytes is shown to be sensitive towards gas passivation effects. Accumulation of gas bubbles disturb the kinetic measurements and lead to undesired mass transport effects. A group of PSI researchers have determined the RM based on the development of a novel characterization technique providing the essential degree of freedom: variation of the water activity. This vapor feed based methodology provided not only for the first time access to characterization of all kinetic parameters and isolation of valid RM and rate determining step but also ensures benchmarking in absence of gas passivation effects. Subsequently, the deduction of a generic relation for gas as well as liquid OER kinetics in polymer electrolyte water electrolysis was obtained. The cited paper gives the reaction order with respect to water (2.07 ± 0.10) and other electro-kinetic parameters for the state-of-the-art OER catalyst IrO2/TiO2 (iridium oxide supported on titanium oxide).