Energy and Environment Research Division
Research at PSI comprises all aspects of human energy use, with the ultimate goal of promoting development towards a sustainable energy supply system. Technologies are being advanced for the utilization of renewable energy sources, low-loss energy storage, efficient conversion, and low emission energy use. Experimental and model-based assessment of these emissions forms the basis of a comprehensive assessment of economic, environmental and social consequences, for both present and future energy supply systems.
Division Head: Prof. Dr. Thomas Justus Schmidt
Energy Briefing Event 2023
The Energy and Environment Division of the Paul Scherrer Institut PSI successfully hosted their second Energy Briefing Event at the Zentrum Paul Klee in Bern. The event focused on the potentials and challenges associated with the production, regulation, and utilization of synthetic fuels. Representatives from WWF, Avenergy, PSI, and BAZL shared their expertise and insights on this topic.
A heartfelt appreciation goes out to Ulrich Koss (Metafuels), Theo Rindlisbacher (BAZL), Christian Bach (Empa), Thomas J. Schmidt (PSI), Thomas Häusler (WWF Switzerland), Daniel Hofer (Avenergy Suisse), and our moderator Stephan Lendi for their invaluable contributions and insightful perspectives.
Energy Briefing Event 2022
On June 28th, 2022, the Energy Divisions (ENE and NES) at PSI hosted their first Energy Briefing Event at the Kursaal in Bern. Knowledgeable voices from industry, research and government shared insights in a dialogue on the feasibility of the Net Zero goal and what next steps are required to achieve this collectively.
A big thank you to Daniela Decurtins (GazEnergy), Particia Sandmeier (Hitachi Energy), Martin Naef (ABB), Pascal Previdoli (BFE), Thomas Schmidt (PSI), Christian Verhoeven (GE), Peter Richner (Empa), Andreas Pautz (PSI) and our Moderator Stephan Lendi for their valuable contributions and insights!
Highlights & News
Polychlorinated biphenyls in glaciers
We present a highly time-resolved historical record of polychlorinated biphenyls (PCBs) from an Alpine ice core (Fiescherhorn glacier, Switzerland). Introduced in the 1940s, PCBs were widely used industrial chemicals. Because of their persistence they are still found in the environment, long after their production phase-out. The Fiescherhorn ice core record covers the entire time period of industrial use of PCBs, that is, 1940?2002. The total concentration of six PCBs varies from 0.5 to 5 ng/L and reveals a temporal trend, with an 8-fold increase from the early 1940s to the peak value in the 1970s.
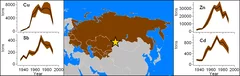
Ice-core based assessment of historical anthropogenic heavy metal (Cd, Cu, Sb, Zn) emissions in the Soviet Union
The development of strategies and policies aiming at the reduction of environmental exposure to air pollution requires the assessment of historical emissions. Although anthropogenic emissions from the extended territory of the Soviet Union (SU) considerably influenced concentrations of heavy metals in the Northern Hemisphere, Pb is the only metal with long-term historical emission estimates for this region available, whereas for selected other metals only single values exist.
Experiments in the clouds – how soot influences the climate
PSI-researcher Martin Gysel receives prestigious European funding (ERC Consolidator Grant) for his studies on the role of soot in cloud formation and global warming.
Particle formation in the atmosphere – further aspect unveiled via the CLOUD experiment at CERN
Clouds consist of cloud droplets that are formed from tiny particles floating in the atmosphere. How these particles develop, however, largely remains a mystery. The formation of particles from amines and sulphuric acid has now been described for the first time à a milestone in atmospheric research.
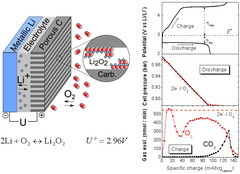
Dosing Differential Electrochemical Mass Spectrometry (D-DEMS) for Li-O2 Batteries
The high-energy rechargeable Li-O2 battery has been subject to intensive research worldwide during the past years. The Li-O2 cell mainly comprises a negative (e.g. Li metal) and positive (e.g. porous carbon) electrode separated by an electronically insulating, but Li+ conducting electrolyte layer. In order to study the cell chemistry, a differential electrochemical mass spectrometry setup based on a set of valves, a pressure sensor and a quadrupole mass spectrometer has been developed.
Effect of surface charge density on the affinity of oxide nanoparticles for the vapor–water interface
Using in-situ X-ray photoelectron spectroscopy at the vapor-water interface, the affinity of nanometer-sized silica colloids to adsorb at the interface is shown to depend on colloid surface charge density. In aqueous suspensions at pH 10 corrected Debye-Hückel theory for surface complexation calculations predict that smaller silica colloids have increased negative surface charge density that originates from enhanced screening of deprotonated silanol groups by counterions in the condensed ion layer.
Chemistry: Ten things we need to know about ice and snow
Understanding the molecular behaviour of frozen water is essential for predicting the future of our planet, says Thorsten Bartels-Rausch.
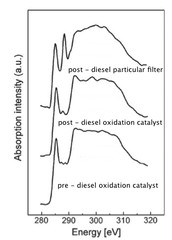
Variations in diesel soot reactivity along the exhaust after-treatment system, based on the morphology and nanostructure of primary soot particles
The reactivity of soot at different sites of the exhaust after-treatment system of a diesel engine (upstream and downstream of the diesel oxidation catalyst (DOC), downstream of the diesel particulate filter (DPF), as well as inside the DPF) was investigated on the basis of morphology and structure of primary soot particles by high resolution transmission electron microscopy (HRTEM). The results indicate that combustion-formed soot particles are susceptible to further transformations of their morphology within the exhaust system.
Supported gold as catalyst for the decomposition of ammonia precursors in the selective catalytic reduction of NOx
Titaniumdioxide supported gold was found to catalyze the hydrolysis of formate-based ammonia precursor compounds which are proposed for the selective catalytic reduction of nitrogen oxides (NOx) in combustion exhaust gas. In contrast to other noble metals, the supported gold does not oxidize the released NH3, while it maintains decomposition of intermediate formic acid.