Suchen
Study of Graphite Cycling in Sulfide Solid Electrolytes
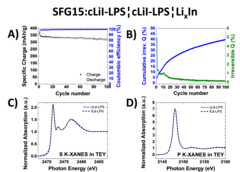
Nowadays, most of the commercial Li-ion batteries employ graphite as the active material in negative electrodes. In the race for the next-generation Li-ion batteries, tremendous research efforts in academia and industry are carried out to replace the current flammable liquid electrolyte with a solid electrolyte, which could improve both, the batteries safety and energy density. Our study investigates two different sulfide-based solid electrolytes, 0.75Li2S-0.25P2S5 (LPS) and 0.3LiI-0.7(0.75Li2S-0.25P2S5), in combination with graphite and discloses the stability of the graphite-solid electrolyte interface. Optimizing the electrode morphology is the key to enhance the rate capability of all-solid-state cells. Using the special tender X-ray range allows chemical characterization of sulfur, phosphor and iodine.
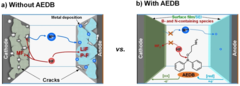
Cross-Talk–Suppressing Electrolyte Additive for Li-ion Batteries
Control of interfacial reactivity at high-voltage is a key to high-energy-density Li-ion batteries. 2-aminoethyldiphenyl borate was investigated as an electrolyte additive to stabilize surface and bulk of both NCM851005 and graphite in the cell with upper cut-off voltage of 4.4 V vs Li+/Li. AEDB almost completely eliminated the “cross-talk” in the cell, by significantly reducing metal leaching from the cathode, preventing their deposition at the anode, and further electrolyte decomposition.
Deciphering the Mechanism of FEC-induced SEI Formation in Li-ion Batteries
Fluoroethylene-carbonate is often referred to as a film-forming electrolyte additive for Li-ion batteries, resulting in high quality Solid–Electrolyte-Interphase on negative electrode, however, the underlying mechanism, even if thought to be known, has been only clarified due to our targeted experimental design, combining systematic electrochemical, chemical and microscopy characterization techniques. We have shown that first the formation of inorganic LiF-rich particles appear and only later the carbonate-rich film is actually formed.
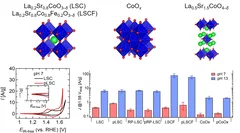
Improving the oxygen evolution reaction activity of Co-based oxides by phosphate functionalization
Our findings disclose that P-functionalization successfully enhances the oxygen evolution reaction (OER) activity of different cobalt-based catalysts (namely, La0.2Sr0.8CoO3–δ, La0.2Sr0.8Co0.8Fe0.2O3–δ, and CoOx) at near-neutral pHs and that both phosphate ion assistance in the OER mechanism and catalyst Co oxidation state can play a role in the enhanced OER activity.
Past Publications
Thin Films and Interfaces
Publications
The publications of our group can also be found at the LCS laboratory. The publications of the individual beamlines are available here SIM and XTREME.
Scientist
Microscopy and Magnetism Group