Structure and Interaction in the pH-Dependent Phase Behavior of Nanoparticle−Protein Systems
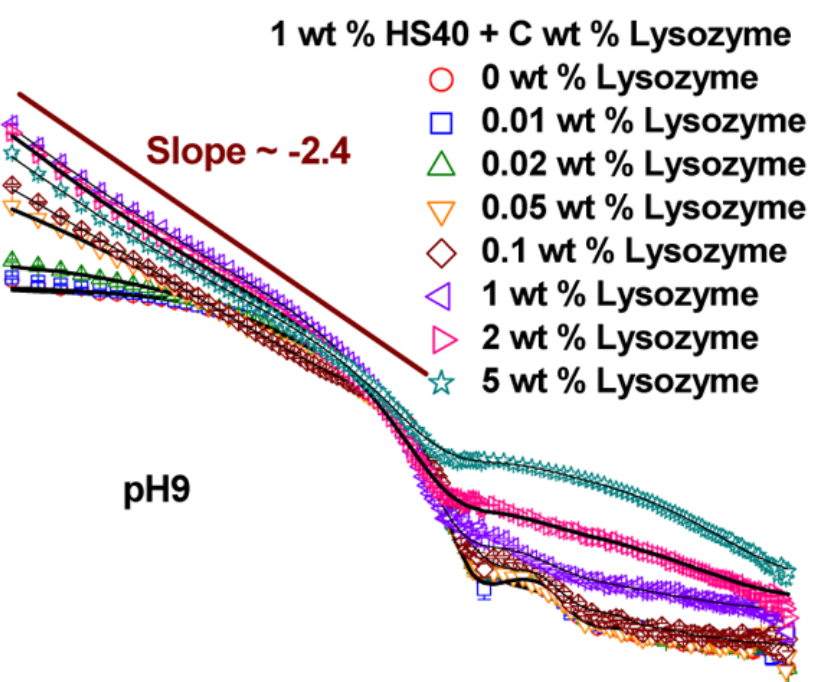
The pH-dependent structure and interaction of anionic silica nanoparticles (diameter 18 nm) with two globular model proteins, lysozyme and bovine serum albumin (BSA), have been studied. Cationic lysozyme adsorbs strongly on the nanoparticles, and the adsorption follows exponential growth as a function of lysozyme concentration, where the saturation value increases as pH approaches the isoelectric point (IEP) of lysozyme. By contrast, irrespective of pH, anionic BSA does not show any adsorption. Despite having a different nature of interactions, both proteins render a similar phase behavior where nanoparticle−protein systems transform from being one-phase (clear) to two-phase (turbid) above a critical protein concentration (CPC). The measurements have been carried out for a fixed concentration of silica nanoparticles (1 wt %) with varying protein concentrations (0-5 wt %)...