Mechano-Genomics Projects
Regulation of genome organization and gene expression by nuclear mechanotransduction during cellular ageing
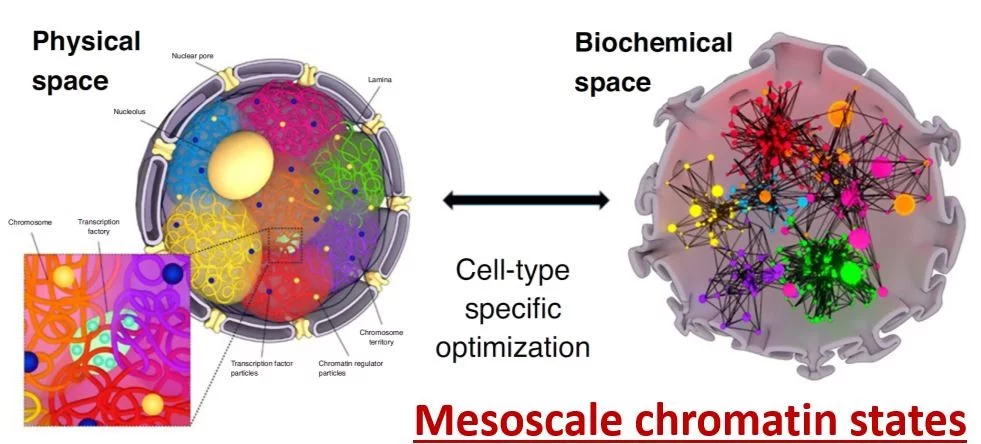
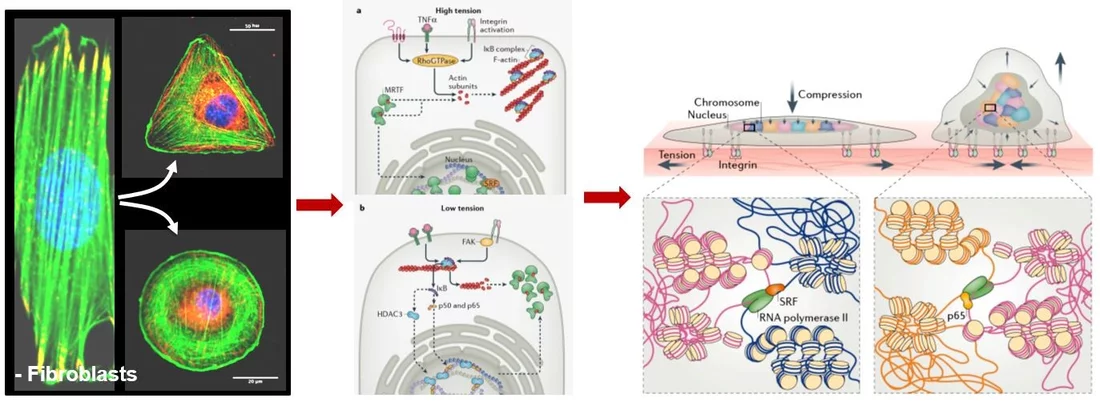
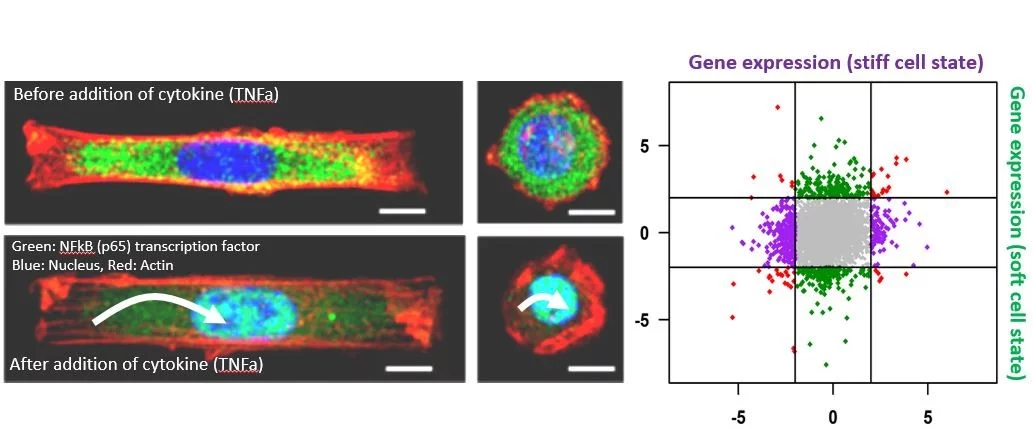
Cells within the tissue microenvironment sense both mechanical and biochemical signals, which modulate cell behavior. We suggest that cell mechanical state dependent extra-cellular signal integration and its coupling to the 3D chromosome organization could be a critical regulator of ageing-dependent gene expression programs. Towards this, we study the activation of specific nuclear mechanotransduction pathways and transcription dependent 3D chromatin reorganization in ageing primary human dermal fibroblasts. We use correlative fluorescence and soft X-ray tomography, combined with chromatin conformation capture data, global histone modification maps, single-cell micromanipulation experiments and RNA-seq to gain mechanistic insights into chromatin reorganization during cellular ageing. In recent experiments, we are also exploring if tissue compression of ageing cells alone could activate youthful mechanotransduction pathways. On the computational side, we are implementing machine learning methods to uncover the functional coupling between 3D chromatin organization and cellular gene regulatory networks. Collectively, these experiments and computational framework will provide a quantitative picture of the mechano-genomics of cellular ageing.
Mechano-genomic regulatory modules in cellular reprogramming and rejuvenation
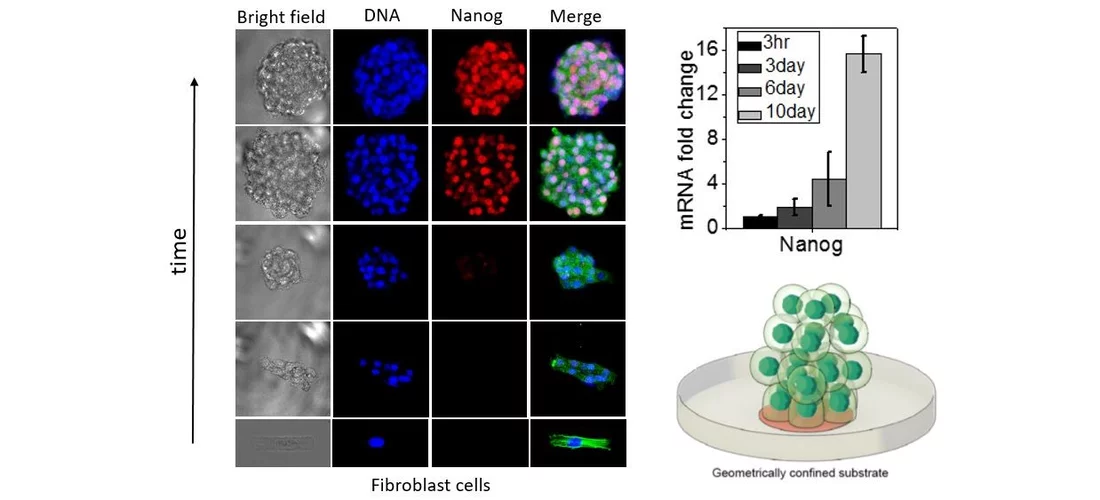
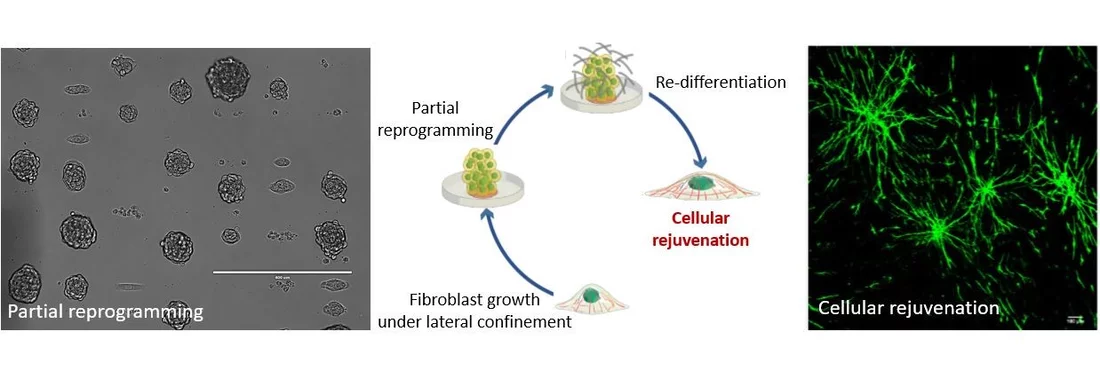
Highlighting the importance of mechanical regulation of genomic programs, we demonstrated that cellular proliferation under confined growth conditions can induce partial reprogramming of fibroblast cells without any exogenous factors. Furthermore, redifferentiation of such partially reprogrammed cells resulted in their rejuvenation in engineered tissues. In recent studies, we are also exploring if ageing related disease cells, such as cancer and fibrotic cells, could be partially reprogramed and rejuvenated. We hypothesize that the partial reprogramming and induced rejuvenation may be intimately linked to higher accessibility of genome regulatory sites and the poised epigenetic states of chromatin regulated by nuclear mechanotransduction. Using time-lapse fluorescence imaging combined with genome-wide analysis and RNA-seq experiments, our ongoing and future experiments are focused on identifying the early molecular pathways that are triggered by the mechanical constraints during partial reprogramming and rejuvenation. On the theoretical side, we are developing robust physical models to gain mechanistic insights on the reprogramming and rejuvenation process. Furthermore, we are also exploring if such partially reprogrammed cells could provide novel cell-based therapies for wound healing and more generally for ageing tissue regeneration and repair.
Single-cell imaging-AI based chromatin biomarkers for cellular ageing and cancer
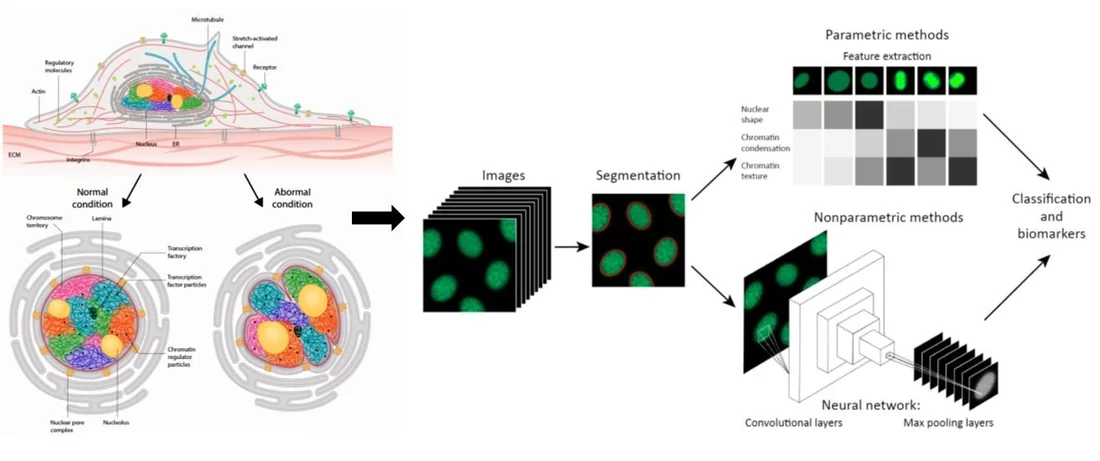
Cell/nuclear mechanical homeostasis is maintained through an elaborate set of cytoskeletal and nuclear proteins that determine the appropriate shape and size of the nucleus and chromatin condensation patterns. Since abnormalities in nuclear and chromatin organization and DNA damage are hallmarks of cellular ageing as well as many ageing related diseases including fibrosis, cancer, and neurodegeneration, we hypothesized that a quantitative analysis of nuclear and chromatin structural alterations could provide a robust functional biomarker to understand the complex interplay between ageing cells and cancer cells in the tissue microenvironment. Towards this, our research on nuclear mechano-genomics combined with fluorescence imaging and machine learning has led to the development of imaging-AI based chromatin biomarkers to analyze cell states and their transitions in the tissue microenvironment. In ongoing work, using human breast tissue microarrays and liquid biopsies, we are exploring if the chromatin biomarkers could provide a quantitative map of ageing tissue microenvironment and tumor progression. Furthermore, for the liquid biopsy analysis, we are developing scalable microfluidic-based devices to improve the sensitivity, efficacy and throughput of our imaging-AI based chromatin biomarkers. Taken together, our studies are aimed at using chromatin structural alterations as quantitative functional biomarkers for cellular ageing, aging related diseases including cancer and neurodegeneration, as well as to assess the efficacy of cancer therapeutic interventions.
Clinical trials
On the medical side, in collaboration with the Center for Proton Therapy at PSI, we have started a clinical trial to analyze if our imaging-AI based chromatin biomarkers of peripheral mononuclear blood cells, obtained from liquid biopsies, could help determine the efficacy and toxicity of proton radiation therapy for treating various cancers in human patients. In addition, together with the local Health Center in Brugg, Switzerland, we have started a clinical trial to assess if our chromatin biomarkers could provide robust methods for early cancer diagnostics. We plan to expand on these ongoing successful clinical trials in future.
Chromatin Imaging Atlas of Blood Cells in Human Cancers - A Public Health Project
We propose to develop a comprehensive chromatin imaging atlas of peripheral blood mononuclear cells (PBMCs) in aging related human diseases. We will use light microscopy combined with AI methods to characterize the subtle alterations in chromatin structure associated with different aging related diseases. Such an imaging atlas will provide new avenues for early diagnostics and tracking of therapeutic interventions. >> more information
Technology transfer
Our research program on mechano-genomics has also resulted in the founding of a company “FOCAL Biosciences” together with Apollo Ventures and PSI/ETH Zurich. The goal of this company is to identify lead compounds for cellular reprogramming in the context of ageing related diseases. FOCAL Biosciences aims to use our group’s knowhow to develop a drug discovery platform using the “mechano-genomic principles underlying fibroblast cell-state transitions and the imaging-AI based chromatin biomarkers”.