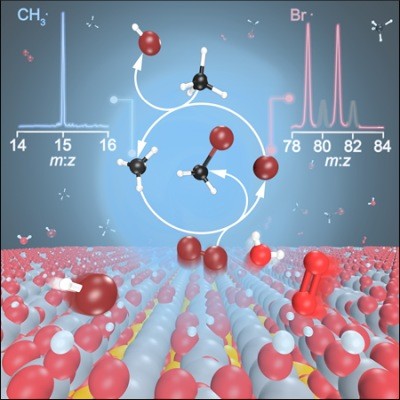
Tracking down radicals in methane oxybromination
Besides being of fundamental interest, understanding the mechanism of methane functionalization is crucial, as it enables transformation of an abundant, cheap, and, potentially, renewable feedstock into commodities. In oxyhalogenation, methane, oxygen and hydrogen halogenides are combined over a catalyst to yield halomethanes. Reaction mechanisms involving surface-catalyzed and gas-phase steps have been proposed to play a pivotal role in methane oxybromination, but due to the lack of experimental evidence, they could not be validated conclusively. Photoelectron photoion coincidence spectroscopy is among the few experimental techniques to characterize reactive intermediates, such as free radicals, prepared by jet-stirred reactors, model flames, pyrolysis, discharge and catalytic reactors, as well as laser photolysis induced chemical reactions.
In cooperation with the Pérez-Ramírez group from ETH Zurich and the Institute of Chemical Research of Catalonia, Barcelona, imaging photoelectron photoion coincidence spectroscopy (iPEPICO) was used at the VUV beamline to provide spectroscopic evidence for the evolution of gaseous methyl and bromine radicals over (VO)2P2O7 and EuOBr catalysts. A the strong correlation between the formation of methyl radicals and the production of bromomethane corroborates the assumption that the mechanisms is not only linked to the surface of the catalyst. Bromine radicals and molecular bromine are generated on the surface, followed by gas-phase methane bromination. The findings emphasize the role of surface and gas-phase steps in halogen-mediated C–H bond activation over heterogeneous catalysts.